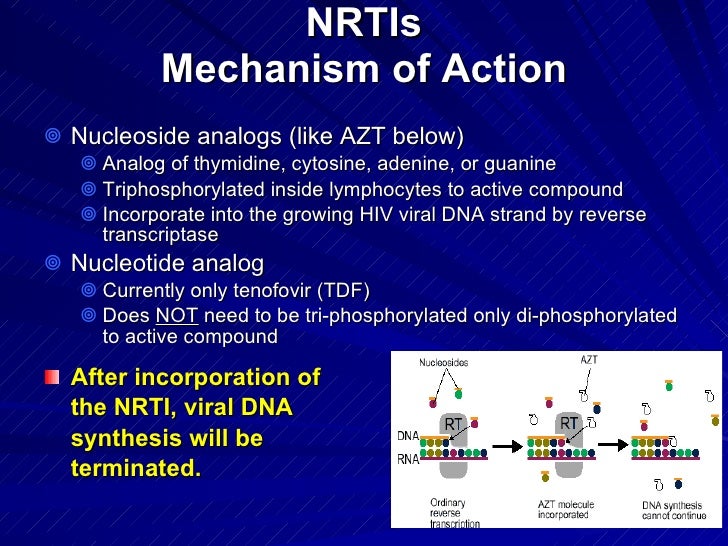
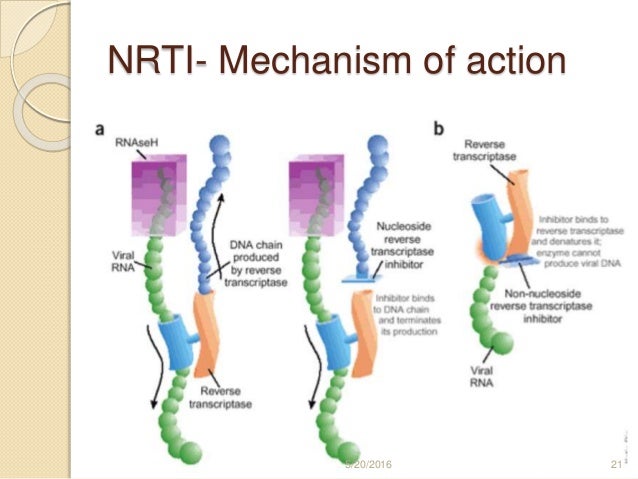
nrti mechanism of action
 HIV Drugs Mode of Action | Immunopaedia
HIV Drugs Mode of Action | ImmunopaediaWarning: The NCBI website requires JavaScript to operate. NCBI Bookshelf. Service of the National Library of Medicine, National Institutes of Health. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan... StatPearls [Internet]. Parth H. Patel; Hassam Zulfiqar.AuthorsAfiliations Last updated: November 30, 2020. Continuous educational activityFacial transcription inhibitors are drugs used in the management and treatment of HIV. He's in the antiretroviral class of drugs. This activity examines the indication, action and contraindications for ICR as a valuable agent in HIV management (and other disorders where appropriate). This activity will highlight the mechanism of action, profile of adverse events and other key factors (e.g., out-of-label usages, dosage, pharmacokinetics, pharmacokinetics, monitoring, relevant interactions) relevant to members of the interprofessional health care team in the (management of HIV patients and related conditions. Objectives:Indictions With the rise in the HIV/AIDS epidemic, many companies have created drugs in the hope of reducing the spread and potentially healing this problem. At the forefront of these drugs are reverse transcription inhibitors. To date, FDA has approved the use of reverse transcription inhibitors for two main viral infections. The first approved use is for the treatment of HIV, specifically HIV-1 strain. The second virus is hepatitis B. Converse transcription inhibitors have also been used for post-exposure prophylaxis when there is concern about the possible infection of HIV patients. Finally, reverse transcription inhibitors are being used to decrease the spread of mother-to-child HIV during pregnancy, as well as childbirth and childbirth. The medicine of choice for the treatment of HIV of the mother during pregnancy is antiretroviral therapy based on zidovudine. Research and testing is currently being conducted to assess the effectiveness of the use of reverse transcription inhibitors for pre-exposure prophylaxis. Studies show that there is a reduction of 67 per cent to 75 per cent of the risk of infection through the use of pre-exposure prophylaxis. While the results have been promising, some have raised strong concerns about the emergence of drug-resistant strains due to the lack of adherence to the pre-exposure prophylaxis protocol by patients. The most important factor in the success of these therapies has been the lack of adherence to the protocol by patients. Mechanism of ActionIn the class of reverse transcription inhibitors are two subclasses of drugs. The first class is nucleoside/nucleotide reverse transcriptase inhibitors, and the second class is non-nucleoside reverse transcription inhibitors. Inverse nucleoside/nucleotide (NRTIs) inhibitors were the first class of antiretroviral drugs to be approved by the FDA. NRTIs are taken as prodrug drugs and must be taken to the host and phosphorylated cell before they become active. Once inside the host cell, cellular kinases will activate the drug. The drug exerts its effect through its structure. NRTIs lacks a 3'-hydroxyl group in the 2'-deoxyribosyl mix and will have a core or nucleotide as a base. Due to the missing 3'hydroxil group, NRTI avoids the formation of a 3'-5'-phosphodiester bond in growing DNA chains and can thus prevent the replication of the virus. An interesting feature of these drugs is that their incorporation during the DNA synthesis dependent on RNA or DNA, which inhibits the production of positive or negative strands of DNA. Inverse transcribase inhibitors (NNRTIs) not nucleosides are the second class of reverse transcription inhibitors. The main action mechanism is by binding the NNRTI to the reverse transcriptase and creating a proximal hydrophobic pocket to the active site. This pocket creates a new spatial configuration of the substrate binding site to reduce the overall activity of the polymerase. By creating a different configuration, DNA synthesis is generally slowed down. Because of NNRTI's non-competitive inhibitive action, it is not effective against reverse HIV-2 transcriptase. Administration The standard of care for HIV treatment requires the combination of NRTI, NNRTI, protease, and integration chain transfer inhibitors. Currently, the recommended regime consists of a "2+1" method where the patient should start at 2 NRTIs followed by an NNRTI, a protease inhibitor (with ritonavir boost), or an integration inhibitor. In terms of the selection of NRTI, various factors such as HIV strain sensitivity, contraindications, adverse reactions and current medicines must be taken into account. The International Antiretroviral Society recommends that treatment begin the day of diagnosis to have the maximum efficacy and slow progression of the disease as quickly as possible. According to IAS, for new HIV infections, recommended initial treatments are integration inhibitors: For pre-exposure prophylaxis cases, recommended treatment regimen: Post-exposure prophylaxis is achievable through any of the following regimens for four weeks: The following list contains some of the approved drugs falling into the NRTI and NNRTI categories of transcribase inhibitors as well. All RTIs come in an oral tablet or solution form with certain medicines that come in other formulations. Nucleoside/Nucleotide Retroceder TranscriptionInhibitorsNucleoside/Nucleoide Retroceder TranscriptionInhibitorsNo-nucleoside Reverse transcription inhibitors While total safe medicines, ICRs have a variety of side effects that should be taken into account when prescribed. Most adverse effects are observed in chronic situations rather than sudden occurrence and associated with each subcategory of the medication. NRTINRTI The most common adverse effect, as well as the most significant adverse effect associated with the use of INA, is mitochondrial toxicity. While new NRTIs have less incidence of mitochondrial toxicity, they still have some risk of causing it. Mythochondrial toxicity due to the use of NRTIs can be manifested as one of the following: myopathy, lipoatrophy, neuropathy and lactic acidosis with or without liver steatosis. Myopathy is more commonly associated with zidovudine and can manifest as proximal muscle tenderness and myalgia. Lipoatrophy (also known as lipodystrophy) is the loss of body fat from the face and limbs. The loss of fat in the areas of cheek, temples and periorbital regions gives patients an empathy appearance. Although this effect correlates strongly with the use of protease inhibitors in HAART, it may also appear in association with the use of stavudine. Peripheral neuropathy associated with NRTI is more common with chronic use of zalcitabine, didanosine and lamivudine. Lactic acidosis occurs more commonly with the use of zidovudine, lamivudine, stavudine and didanosine. Hepatic steatosis usually occurs accompanied by lactic acidosis due to the decrease in mitochondrial beta-oxidation of fatty acids resulting in sterified triglycerides that accumulate in the liver. NNRTINNRTI Compared to NRTIs, NNRTIs have been correlated with less adverse effects. As a group, all NNRTIs are known to cause rash. The most severe of these eruptions are Stevens-Johnson syndrome, as well as toxic epidermal necrolysis. In addition to the complications of dermatitis by using NNRTIs, some NNRTIs are known to cause other adverse effects. In comparison, nevirapin has been shown to cause significant transaminitis. Efavirenz, unlike the other NNRTIs, has shown CNS alterations. These CNS effects include mood problems, insomnia and disturbing dreams. Finally, it is known that delavirdine causes neutropenia when administered with zidovudine. Contraindications While reverse transcription inhibitors are generally safe to use, there are contraindications to prevent serious adverse effects associated with their use. First, a previous history of hypersensitivity to ICRs is a contraindication to its use. If a patient has had an adverse reaction to an RTI, he must suspend the medication and a different prescribed agent. Abacavir is explicitly associated with a hypersensitivity reaction that threatens life in 5% of patients. Specifically, patients who carry the HLA-B*5701 allele have the greatest chance to experience a severe reaction to the medication. Patients with HLA-DR7 and HLA-DQ3 should also avoid the use of abacavir. Patients with high or abnormal LTA or AST should avoid the use of nevirapin-based antiretroviral therapy due to severe hepatotoxicity and hepatotoxicity associated with rash. Didanosine has also shown a clinically significant interaction with the medication when taken concomitantly with alopurinol. MonitoringMonitoring for RTIs and HAART, in general, is essential due to the disastrous effects of medication noncompliance and suboptimal therapy. Surveillance is performed mainly through assessments such as CD4 count and viral load. The CD4 count is useful for measuring because it is the main goal of HIV. Patients with low CD4 counts may be expected to have severe immunodeficiencies leading to opportunistic infections. Viral charge looks at how many copies of HIV RNA is present in the patient. The higher the viral load, the greater the presence of HIV infection. The current IAS recommendations indicate that patients should have a level of HIV RNA (viral charge) within six weeks of starting HAART to evaluate the adhesion and tolerability of therapy, while observing that the proper suppression of NR may take up to 24 weeks. Once the viral load has fallen below 50 copies/mL, the recommendation is to repeat the viral load every three months until it reaches a year of suppression. After one year of successfully suppressed viral load, the patient may have their HIV RNA levels measured every six months. IAS recommends checking the CD4 cell count every six months until the levels are above 250/microliter for at least one year. After achieving these objectives, the measurement of the CD4 account can cease. Unless the patient is subjected to steroid or immunosuppressive treatments, or the patient experiences HAART failure, the CD4 count does not need to be measured. The definition of HAART failure is IAS as a level of HIV RNA over 200 copies/mL at least two consecutive measurements. After diagnosing HAART's failure, the recommendation is a re-evaluation of the HIV genotype and drug susceptibility, and making changes in the patient's regime. When talking about the HAART fault, it is important to consider drug resistance as a causative agent. Drug resistance is a serious complication that should be considered and associated with poor drug compliance. In addition, factors such as the cost of treatment, accessibility to medicines and access to adequate follow-up all play an important factor in the upbringing of resistance to standard HIV regimes. Studies have shown that low adherence and interruptions in treatment for more than two consecutive days in the first three months with NNRTI and NRTI-based regimes increased the risk of resistance in the first six months of treatment. Studies have shown that while both measures help to determine the effectiveness of treatment, each variable offers different views on the condition of the patient. The CD4 count, when presented at a normal level through treatment, is associated with a lower risk of mortality. When the viral load becomes undetectable, it means that the drug regime in which the patient is working is most effectively. In addition, monitoring of viral load is considered to be a better predictor of HIV progression to AIDS than CD4. ToxicityToxicity to RTIs occurs mainly through adverse reactions with which the patient may present (see above for a list of severe reactions). Patients should understand the serious adverse effects that may cause the drugs that make up their HAART regime. If a patient has an adverse effect, then the medication should be interrupted and replaced by another of the same inverse transcription inhibitor subclass. Specifically for stavudine, studies suggest that changing to abacavir will help improve lipodistrophy and maintain the effectiveness of the optimal regime. All other drugs that cause adverse reactions need to change to a drug that your specific HIV genotype is susceptible to. Improve the results of the health care team Patients in STDs, and more importantly, HAART, should have close follow-up with the primary doctor who cares for them. Doctors should work closely with nurses to follow the appropriate follow-up of patients to ensure optimal therapy provision along with the search for possible adverse effects. Doctors can also work closely with pharmacists to help optimize treatment and give the best medications to patients to achieve optimal therapy; the pharmacist can consult the best combinations of agents, as well as optimal dosage and administration. The nursing and pharmacy should emphasize the importance of strict patient compliance, since lack of compliance can be devastating for therapy and, subsequently, their life. The attention of the doctor who immediately describes compliance must be drawn. All members of the inter-professional medical care team are responsible for providing the best care to their patients, as well as for finding adverse effects. An interprofessional team will result in the best results. [Level 5]Continuing Education / Review QuestionsReferences This book is distributed under the terms of the Creative Commons 4.0 International License (), which allows use, duplication, adaptation, distribution and reproduction in any medium or format, provided that you give appropriate credit to the original author(s) and source, a link to the Creative Commons license is provided, and the changes made are indicated. ViewsIn this PageRelated information Similar products in PubMed Recent activityYour navigation activity is empty. The activity recording is off. , 8600 Rockville Pike, Bethesda MD, 20894 USA

A) Structures of lamivudine and other NRTIs used in contemporary HIV... | Download Scientific Diagram

Nucleotide Reverse Transcriptase Inhibitors: A Thorough Review, Present Status and Future Perspective as HIV Therapeutics | Bentham Science
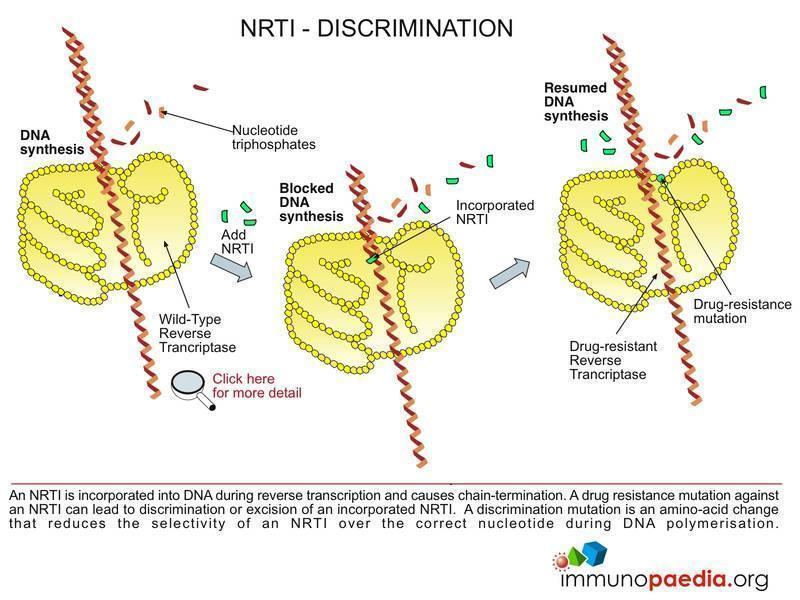
HIV Drugs Mode of Action | Immunopaedia
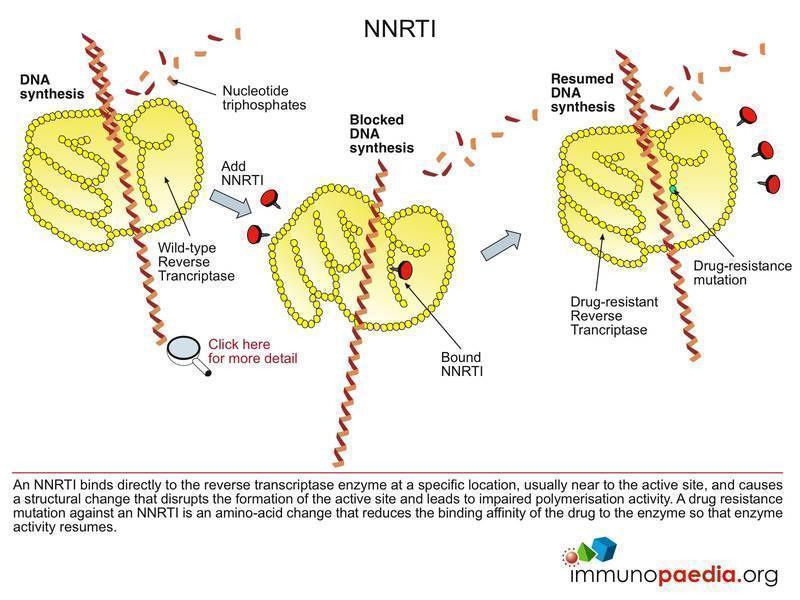
HIV Drugs Mode of Action | Immunopaedia
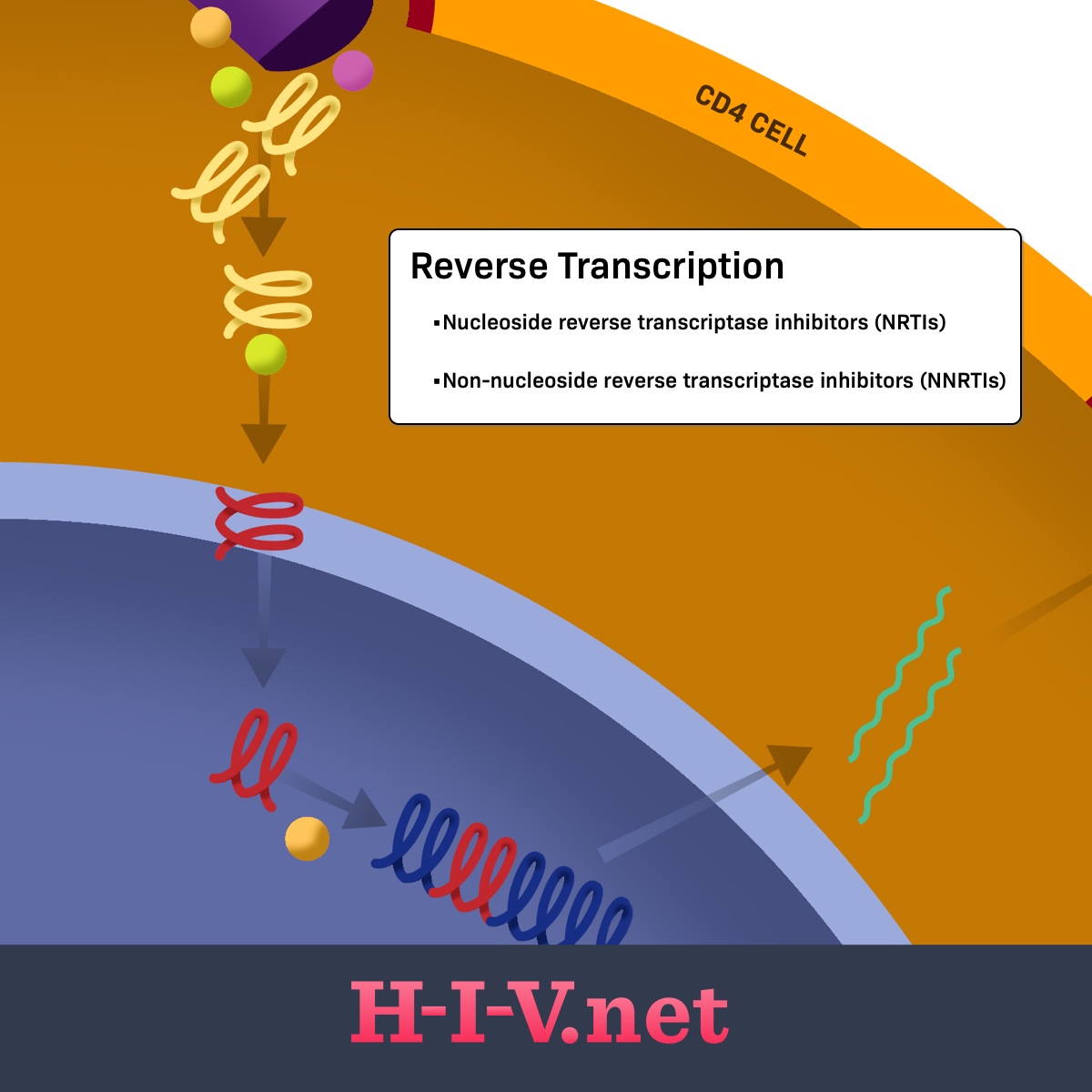
Nucleoside Reverse Transcriptase Inhibitors (NRTIs) | HIV
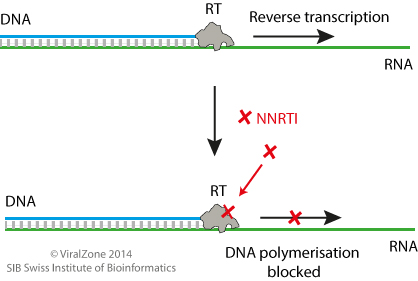
Non-Nucleoside Reverse Transcriptase Inhibitors ~ ViralZone
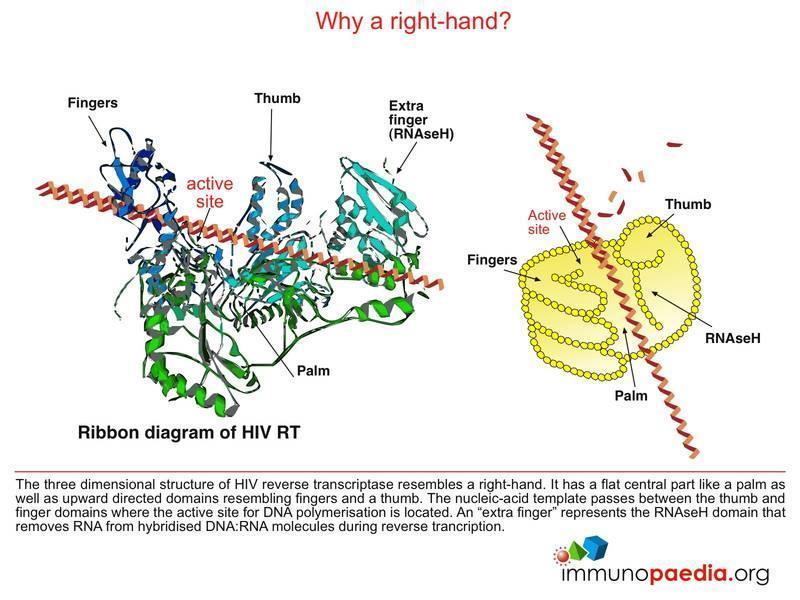
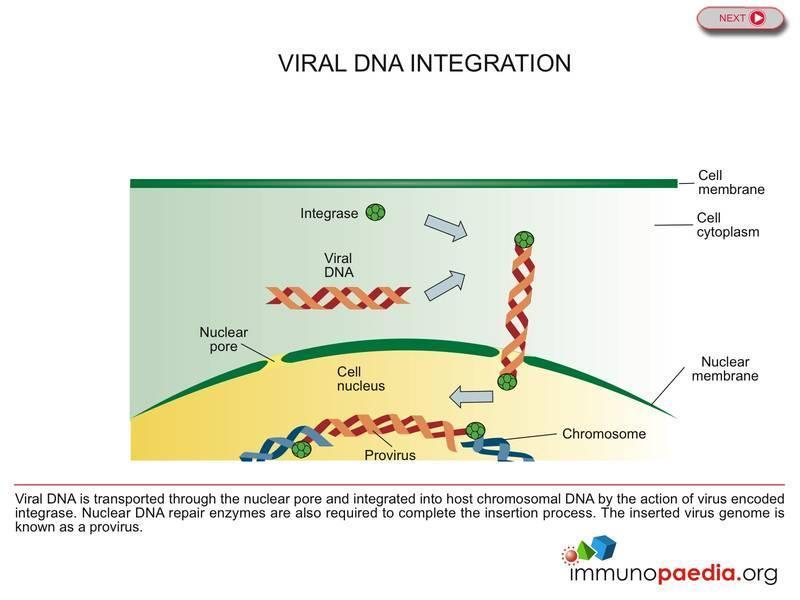
HIV Drugs Mode of Action | Immunopaedia

Core Concepts - Antiretroviral Medications and Initial Therapy - Antiretroviral Therapy - National HIV Curriculum
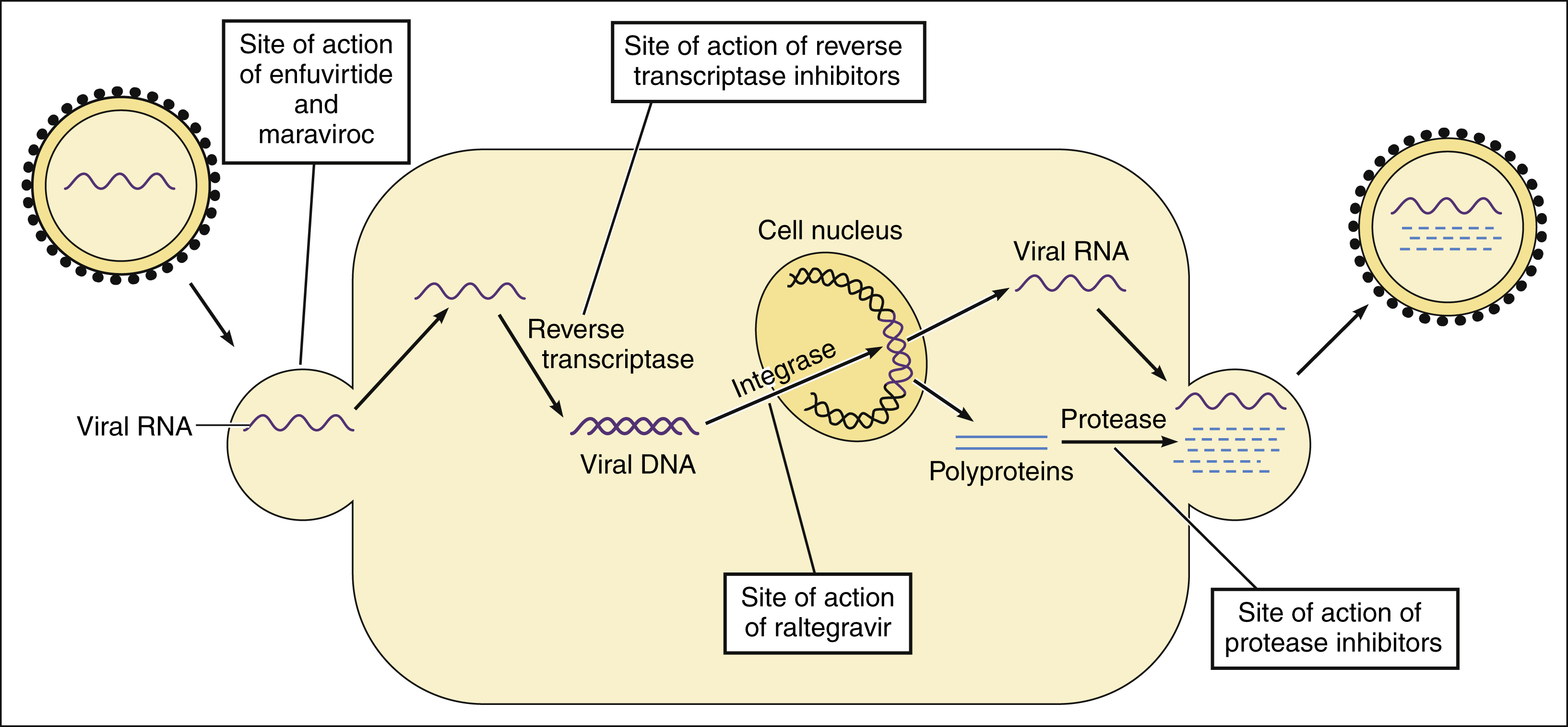
Antiretrovirals

Discovery and development of nucleoside and nucleotide reverse-transcriptase inhibitors - Wikipedia
Antiviral Drugs | Basicmedical Key

آشنایی با داروهای مصرفی در ایدز - ppt download
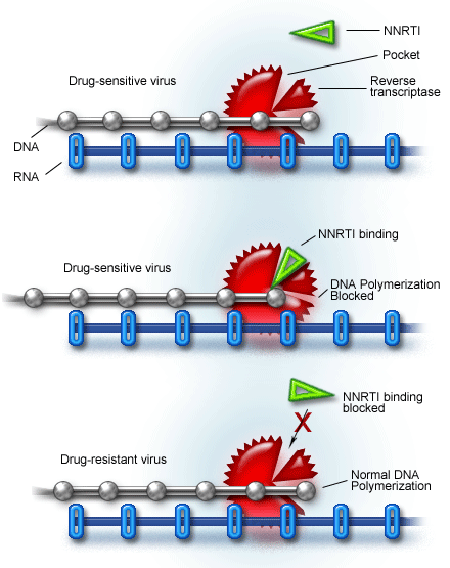
NNRTIs: The Next Generation Approaches

mechanism of action of NNRTI's | Download Scientific Diagram
![hiv_haart [TUSOM | Pharmwiki] hiv_haart [TUSOM | Pharmwiki]](https://tmedweb.tulane.edu/pharmwiki/lib/exe/fetch.php/nrtitoxicity.png?w=700&tok=cba126)
hiv_haart [TUSOM | Pharmwiki]
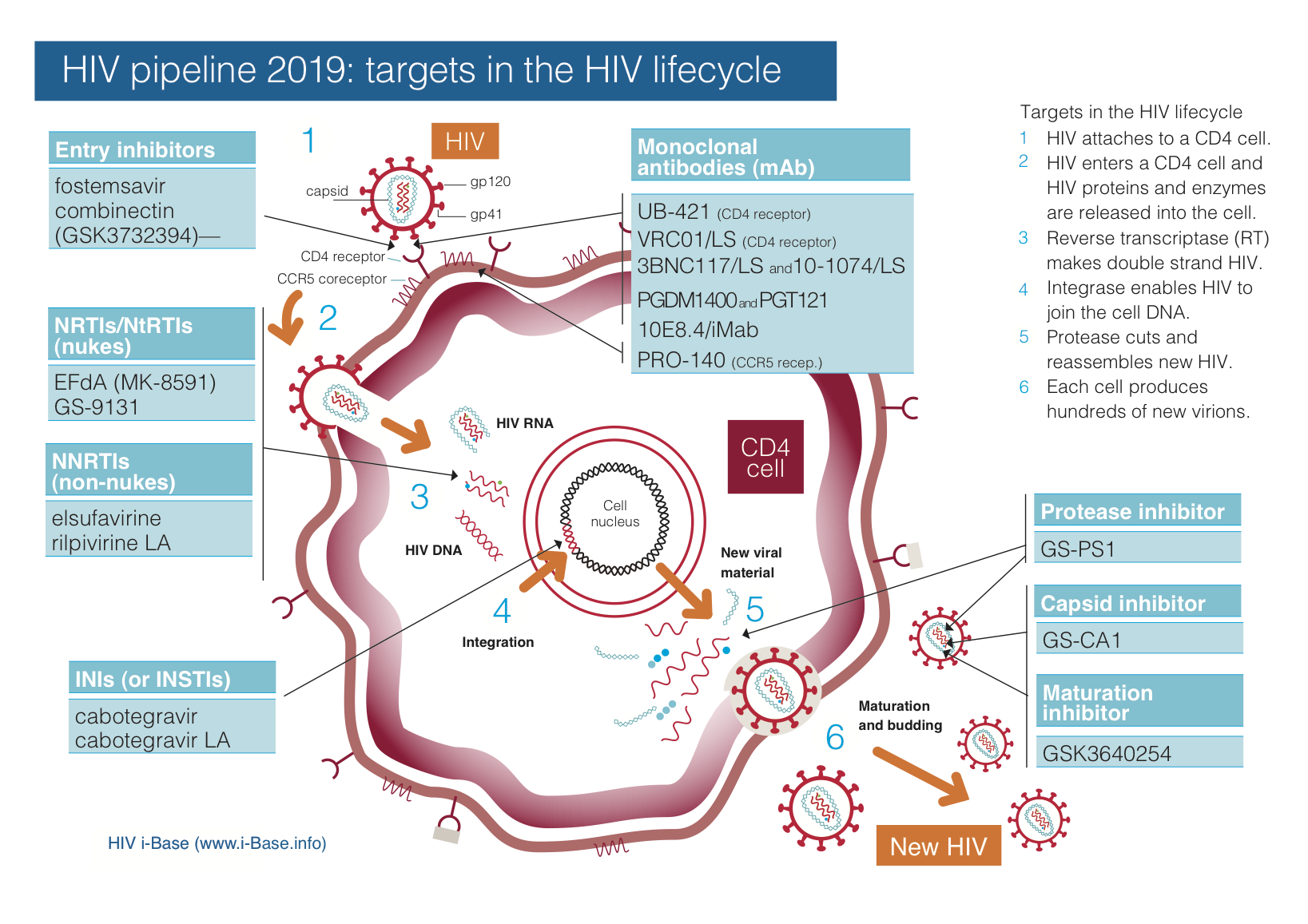
HIV pipeline report 2019 | HTB | HIV i-Base

Hepatitis B virus reverse transcriptase - Target of current antiviral therapy and future drug development. - Abstract - Europe PMC

Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-γ inhibition: Trends in Pharmacological Sciences

A) HIV-1 infection pathway and (B) Antiretroviral drug (ARV) action... | Download Scientific Diagram
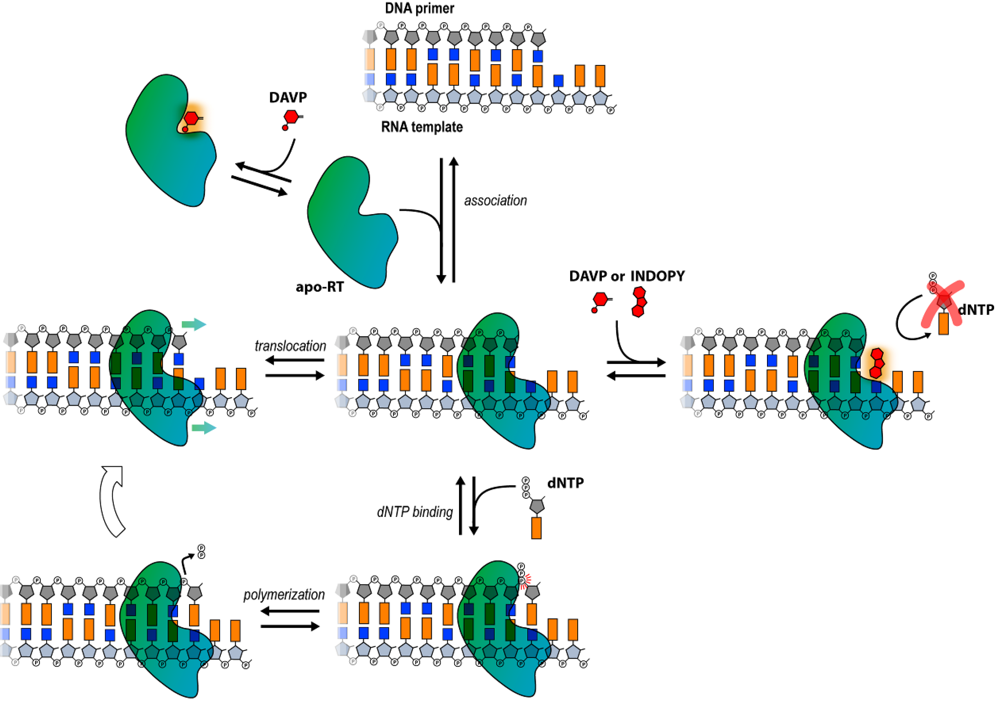
Viruses | Free Full-Text | HIV-1 RT Inhibitors with a Novel Mechanism of Action: NNRTIs that Compete with the Nucleotide Substrate | HTML

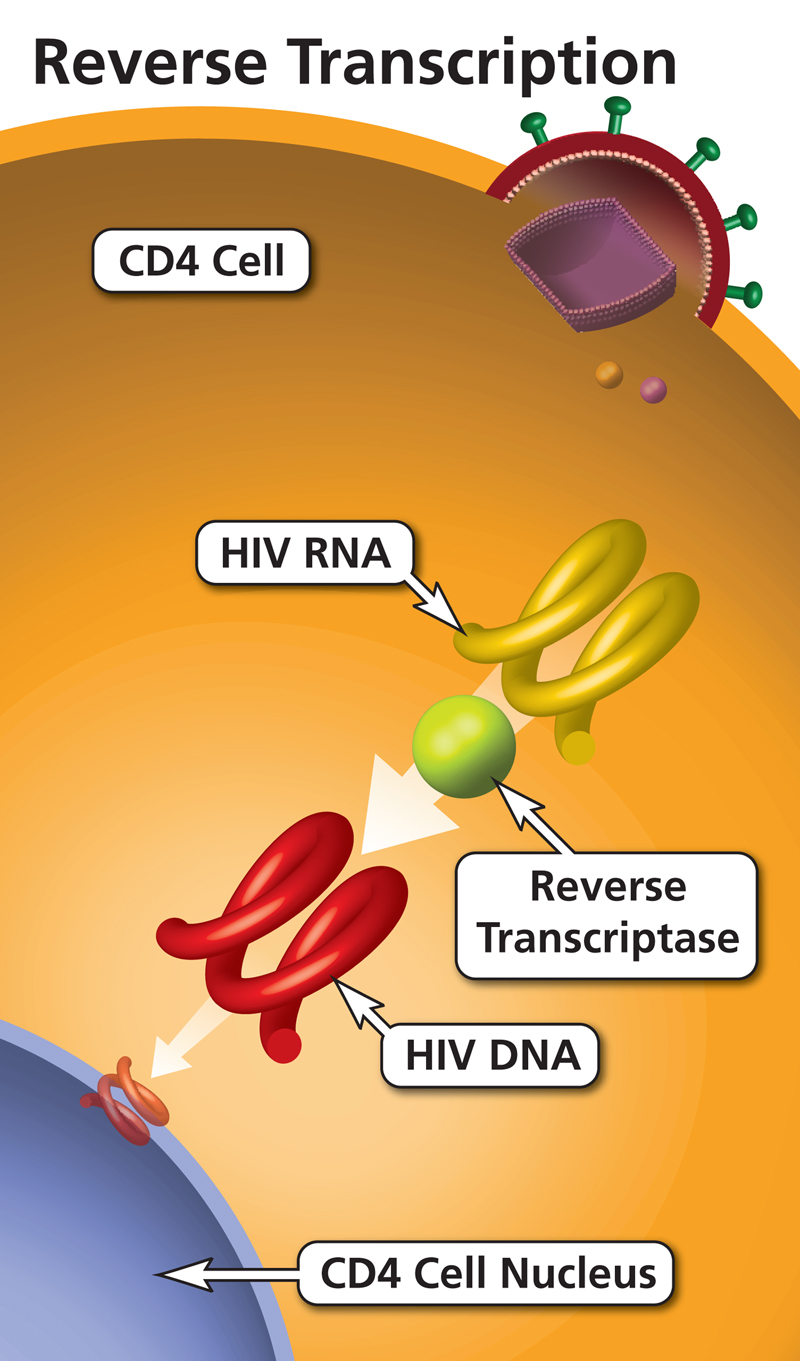
Nucleoside reverse transcriptase inhibitors (NRTIs) - Osmosis

Mechanisms of NRTI resistance. (A) Nucleotide excision. Mutations in... | Download Scientific Diagram

BIKTARVY® Mechanism of Action | Official HCP Site
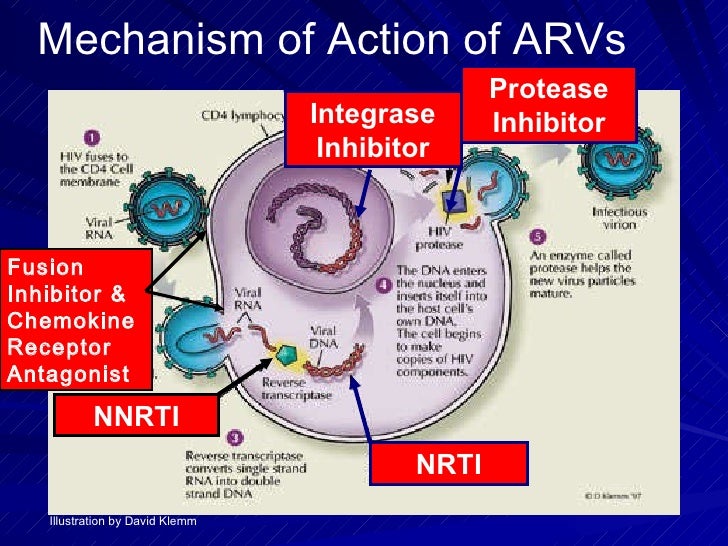
Antiretrovirals

Some mechanisms are proposed to explain the effects of nucleoside... | Download Scientific Diagram
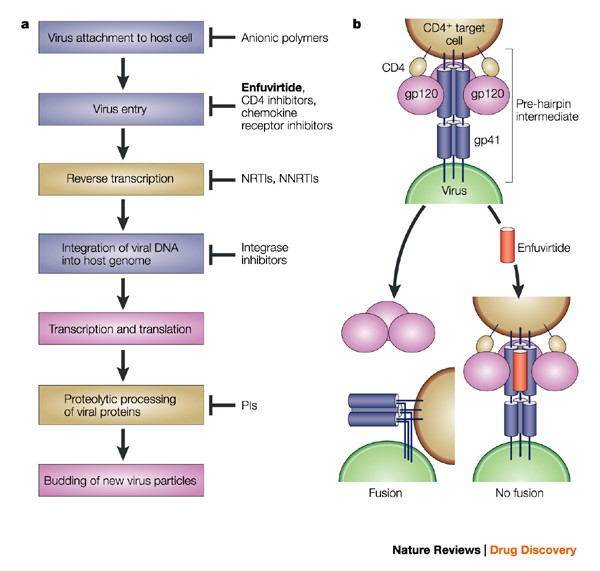
Infectious diseases | Nature Reviews Drug Discovery

Mechanisms of C-terminal domain NRTI resistance. During reverse... | Download Scientific Diagram

rugs, Mechanism Of Action, Adverse Effects, 2007
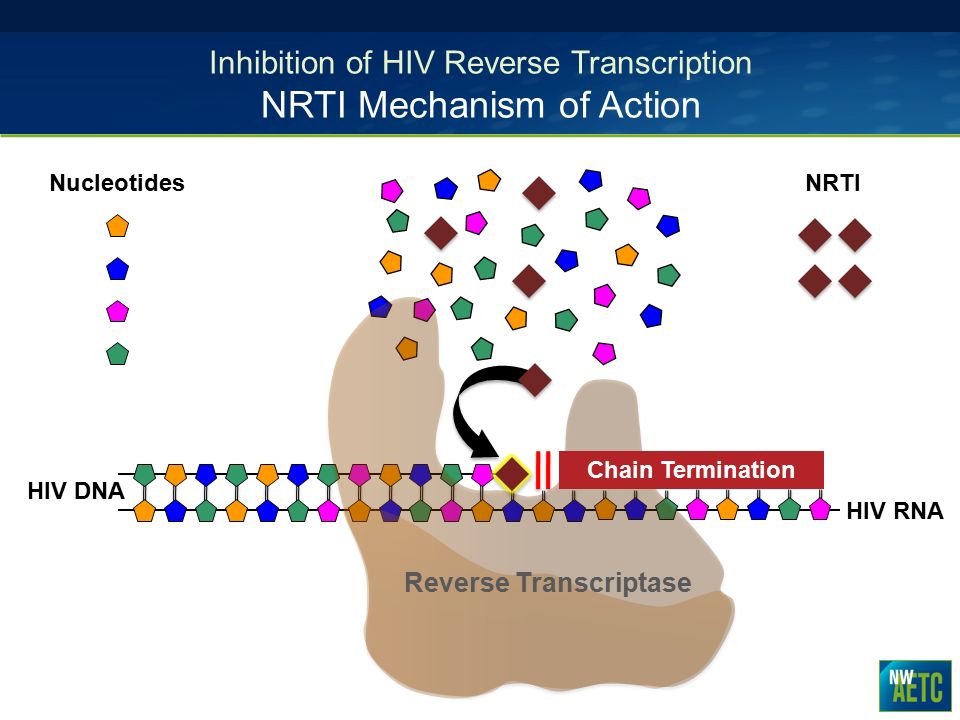
Management of NRTI Resistance - ppt video online download
sgugenetics / HIV developing drugs
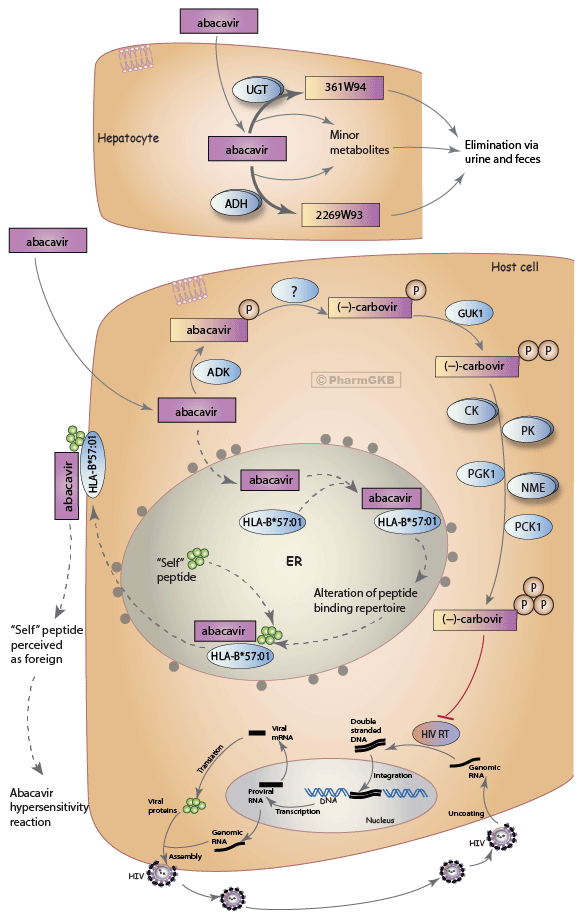
Abacavir Pathway, Pharmacokinetics/Pharmacodynamics

Antiretroviral drugs

BIKTARVY® Mechanism of Action | Official HCP Site
Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI) | ClinicalInfo

Mode of action of non-nucleoside reverse transcriptase inhibitor... | Download Scientific Diagram
HIV/ AIDS: Recent advances 2016

Antiretroviral Drugs: How HIV Medications Work - Video & Lesson Transcript | Study.com

Core Concepts - Antiretroviral Medications and Initial Therapy - Antiretroviral Therapy - National HIV Curriculum

Figure 1 | Topical Prophylaxis for HIV Prevention in Women: Becoming a Reality | SpringerLink

Antiretroviral Therapy: Drugs, Mechanism of Action, Adverse Effects - ppt download
Posting Komentar untuk "nrti mechanism of action"